Abstract
Background: Sickle cell disease (SCD) is characterized by painful vaso-occlusive events (VOEs), progressive vasculopathy, hemolytic anemia, and organ damage resulting in frequent hospitalizations and decreased quality of life (QoL). The ongoing phase 1/2 HGB-206 study is evaluating the efficacy and safety of LentiGlobin for SCD (bb1111) gene therapy and has demonstrated complete resolution of severe VOEs, near-normalization of key hemolysis markers, and normalization of total hemoglobin up to 24 months post-LentiGlobin infusion in Group C. In addition, LentiGlobin for SCD also showed clinically meaningful improvements in QoL in adult patients. Here we present patient-reported QoL outcomes up to 24 months post-LentiGlobin infusion in Group C of the HGB-206 study.
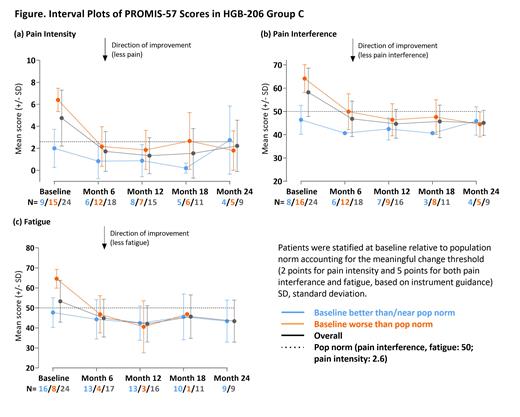
Methods: Patients (≥12-≤50 years of age) with severe SCD and recurrent severe VOEs underwent plerixafor mobilization and apheresis followed by myeloablative busulfan conditioning and LentiGlobin infusion. In addition to laboratory and clinical assessments, patients were monitored for patient-reported outcomes (PROs) at baseline and every 6 months post-infusion through Month 24 using PRO Measurement Information System (PROMIS)-57, a QoL-monitoring tool that has been validated in SCD. PROMIS-57 uses a collection of short forms to assess 7 PROMIS domains of relevance to patients' physical, mental, and social wellbeing (Depression, Anxiety, Pain Interference, Fatigue, Sleep Disturbance, Physical Function, Satisfaction with Participation in Social Roles) as well as a 0-10 Pain Intensity numeric rating scale (NRS). Available data were analyzed for 25/35 Group C patients (median age: 25 [19-38]; 40% female) who completed PROMIS-57 assessments at baseline with up to 24 months of follow-up as of February 17, 2021. For each domain, patients from the overall population were stratified into 2 subgroups depending on whether their baseline score was "better or near" or "worse" than the population norm, to account for potential differences over time in QoL changes relative to baseline status, and their means and standard deviations were plotted over time. The US general population norm is a standardized T-score of 50 for all domains and a 2.6 for Pain Intensity NRS. Meaningful change, a minimal response deemed meaningful to the patient, was interpreted at group level as at least a 5-point change from baseline for domains and a 2-point change for the NRS, as based on PROMIS guidelines and published literature.
Results: In general, patients with baseline scores "worse" than the population norm reported improvements in all domains at Month 6 up to Month 24. Of note, mean pain interference decreased from 64.2 (n=16) to 44.5 (n=5), pain intensity decreased from 6.5 (n=15) to 1.8 (n=5) from baseline to Month 24 and fatigue decreased from 64.6 (n=8) to 46.9 (n=1) from baseline to Month 18, respectively (Figure). In patients with baseline scores "better or near" population norm, scores generally remained stable through Month 24. For example, mean pain interference scores were 46.4 (n=8) and 45.9 (n=4), pain intensity scores were 2.0 (n=9) and 2.8 (n=4), and fatigue scores were 47.7 (n=16) and 43.4 (n=9) at baseline and last visit, respectively. When considering mean differences, values for patients overall showed meaningful change in all domains and the Pain Intensity NRS, with the exception of Anxiety. Expected variability due to the small sample size limits interpretation. Mean values over time will be presented for all domains.
Summary: In Group C of the HGB-206 study, patients with baseline scores "worse" than population norm had improved scores across all PROMIS-57 domains that were established early post-infusion and sustained up to Month 24. Patients with baseline scores "better or near" population norm were stable, and not worse up to Month 24. Mean values for patients overall indicate meaningful change at latest follow-up for all domains and Pain Intensity NRS, with the exception of Anxiety. These data show LentiGlobin for SCD not only improved hematologic parameters and resulted in complete resolution of severe VOEs, as presented elsewhere, but also provide sustained and clinically meaningful QoL benefit for patients. Continued follow-up and analysis of patient-reported outcomes is needed to evaluate the long-term impact of LentiGlobin for SCD.
Walters: Vertex pharmaceuticals: Consultancy; BioLabs, Inc: Consultancy; Ensoma, Inc.: Consultancy; AllCells, Inc: Consultancy. Kwiatkowski: Silence Therapeutics: Consultancy; Sangamo: Research Funding; Bioverativ: Research Funding; Apopharma: Research Funding; Bristol Myers Squibb: Consultancy; Imara: Consultancy, Research Funding; Celgene: Consultancy; Agios: Consultancy; bluebird bio,Inc.: Consultancy, Research Funding. Aygun: Patient Centered Outcomes Research Institute: Research Funding; bluebird bio, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; National Heart, Lung, Blood Institute: Research Funding; National Institute of Nursing Research: Research Funding; Global Blood Therapeutics: Consultancy. Gallagher: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Zhang: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Ho: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Thompson: Graphite Bio: Research Funding; Agios: Consultancy; Novartis: Research Funding; Global Blood Therapeutics: Current equity holder in publicly-traded company; Beam: Consultancy; CRISPR Therapeutics: Research Funding; Celgene/BMS: Consultancy, Research Funding; Biomarin: Research Funding; Baxalta: Research Funding; bluebird bio, Inc.: Consultancy, Research Funding; Vertex: Research Funding; Editas: Research Funding. Kanter: Fulcrum Therapeutics, Inc.: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Forma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beam: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Graphite Bio: Consultancy; GuidePoint Global: Honoraria; Fulcrum Tx: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal